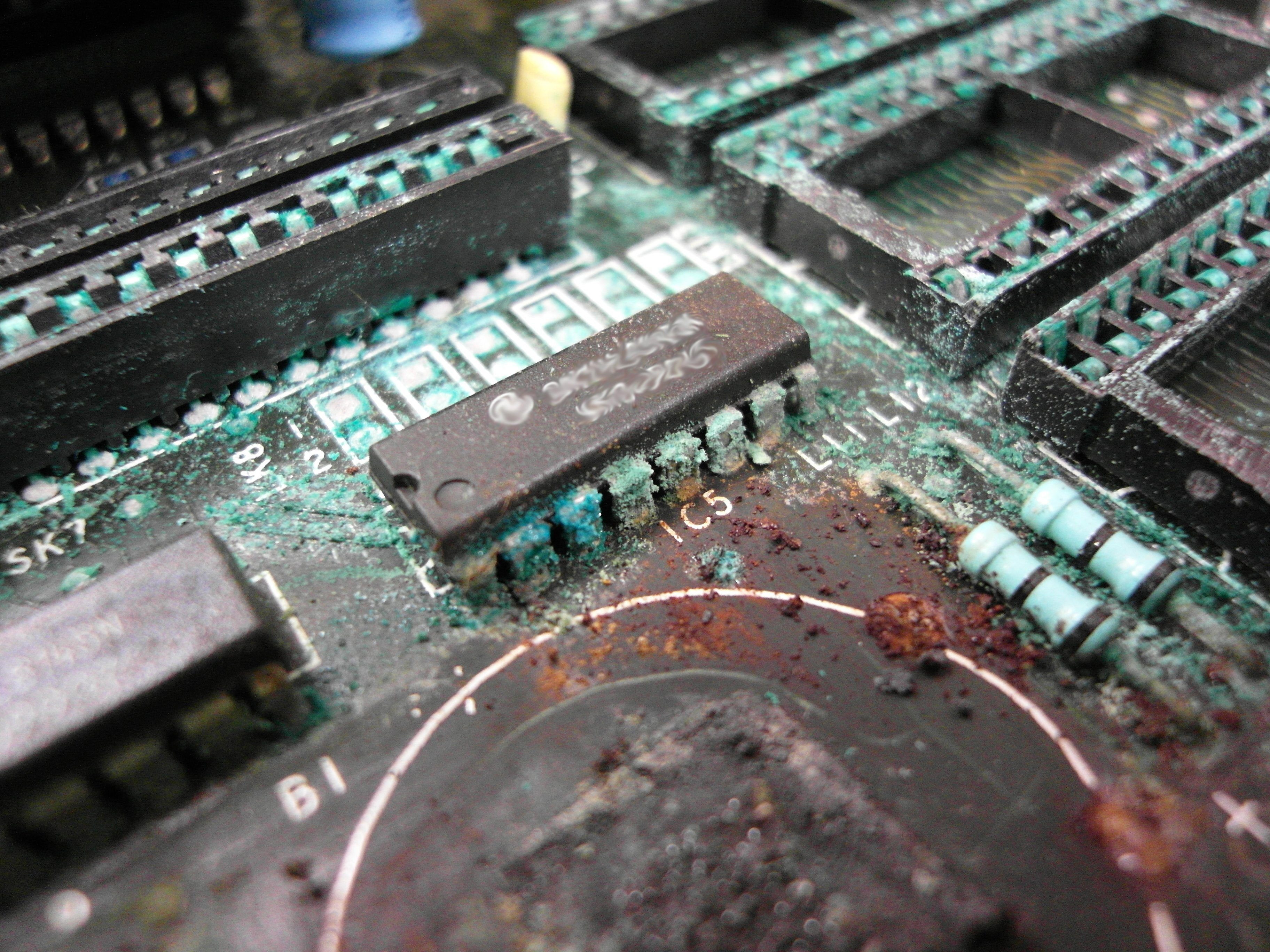
Galvanic reaction is a fascinating phenomenon in materials science that plays a critical role in the durability and reliability of our electronic devices. Let’s take a closer look at what it is, how it works, and why it matters.
What is Galvanic Reaction?
When two different metals come into contact in the presence of an electrolyte (like moisture), an electrochemical process called galvanic reaction can occur. This process causes one of the metals (the more active one) to corrode faster than it normally would, while the other metal (the less active one) is protected.
The driving force behind this reaction is the difference in the electrochemical potential between the two metals. In essence, one metal acts as an anode (where oxidation occurs and metal ions are released), and the other acts as a cathode (where reduction occurs). The flow of electrons from the anode to the cathode through the electrolyte completes the circuit, resulting in the corrosion of the anodic metal.
Why is This Important?
1. Durability: Understanding and mitigating galvanic reactions helps us select the right materials to ensure the longevity of electronic devices. This means fewer breakdowns and replacements, which translates to cost savings and less environmental waste.
2. Reliability: Proper material selection and design can prevent unexpected failures in electronic components. This is especially crucial in high-stakes industries such as aerospace, medical devices, and automotive engineering, where reliability is non-negotiable.
3. Maintenance: By preventing galvanic corrosion, we reduce maintenance needs, leading to more efficient and hassle-free operation of our gadgets and systems.
Considerations:
It is important to know many components come tin plated, however many suppliers offer options. When adding components to printed circuitry, a conductive epoxy is laid down to mate components to the circuitry. A tin plated component on silver epoxy would be a sure-fire way to risk a galvanic corrosion. Another downfall from this reaction is that it would also create non-conductive oxide at the joints, which could then lead to failed components.
This is obviously an exaggerated picture but you want to avoid any (and all) corrosion & oxidization!
Preventing Galvanic Reactions:
1. Material Selection: Choosing metals that are close together on the galvanic series can minimize the potential for galvanic corrosion.
Using gold or silver plated components would prevent this reaction; a noble metal on the component contact and the noble metal in the silver epoxy. Two noble metals “play” together much nicer.
This explains why many LEDs are gold plated, serving two important functions:
- electrical performance
- the ability to “mate with the base circuit”
2. Isolation: Applying coatings or barriers between different metals can prevent direct contact, reducing the likelihood of a galvanic reaction.
3. Environmental Control: Keeping devices dry and free from corrosive substances can further mitigate the risk of galvanic corrosion.
Xymox’s Approach to Galvanic Reaction:
At Xymox, we prioritize understanding and addressing these scientific principles to design and manufacture products that stand the test of time. Our expertise in materials science allows us to develop solutions that mitigate the risks associated with galvanic reactions. We make our conductive epoxy in-house; this gives us complete control of cost, volume, viscosity, and resistance. By leveraging our in-depth knowledge and innovative techniques, we ensure that your devices remain reliable and durable.
Understanding galvanic reactions is essential for creating long-lasting and reliable electronic products. At Xymox, we take pride in our ability to apply this knowledge to enhance the performance and lifespan of the technologies we develop. By staying informed and investing in quality, we help keep your technology running smoothly.